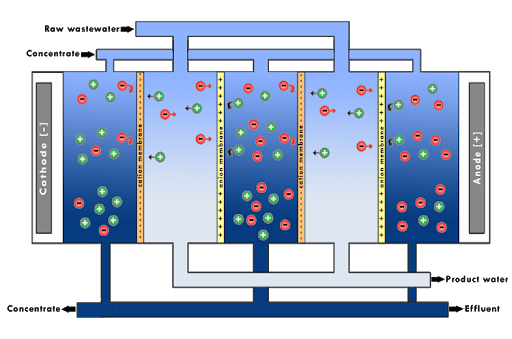
Electrodialysis is a membrane process that uses alternating Anion–selective membranes (AMs) and Cation-selective membranes (CMs), placed between an Anode (+) and a Cathode (-). Due to the applied electric field, anions will move towards the Anode and cations will move towards the Cathode. Anions are stopped by the CMs and the cations by the AMs, creating a process flow with low ion concentration (Dilutant) and a process flow with high ion concentration (Concentrate). A pair of a CM and a AM and both areas between these membranes is a Cell Pair. A Cell Pair is the basis unit of a stack, and is repeated “(n)” times. The number of cell pairs in an actual stack varies depending on the electrodialysis system, with as many as 600 cell pairs in a typical industry-scale system. In electrodialysis suspended solids which carry positive or negative electrical charges can increase the resistance of the membrane dramatically, are deposited on the membrane surface. However, in electrodialysis the problem has been eliminated to a large extent by reversing in certain time intervals the polarity of the applied electrical potential which results in a removal of charged particles that have been precipitated on the membranes. This technique is referred to as electrodialysis reversal (EDR).
EDR Process
In each EDR stack there are two electrodes on the outer side which are submerged in a watery salt solution that is able to conduct electrical current and allows for an electrical field to be placed around the stack. The salt solution is pumped around in order to maintain the ion balance. Because salt solution (feed current) is also found between the ion exchange membranes, the electrical field will result in ion transport. In the spaces between electrodes, marked as “Dilutant”, the cations will diffuse through the CM to the negative electrode (cathode) while the anions will diffuse through the AM to the positive electrode (anode). The ions leaving the dilutant feed are moving to the neighbouring concentrate feed chamber which leads to a drop in concentration of ions in the diluent chambers of the EDR process. In the concentrate chambers, the cations will try to move to the negative electrode but they will be blocked by the AM and the anions will try to move to the positive electrode but will be 3 Christos Charisiadis – ED/ZLD text blocked by the CM. This leads to an increase of their respective concentrations in the concentrate chambers. In EDR, the voltage at the electrodes is reversed every 30 – 60 min which reverses also the direction of ion transport and causes the removal from the membrane surface of electrically charged substances that may cause serious, perhaps irreparable damage.
Remove specific Ions with Selectivity Mode.
- Conventional EDR pulls all ions across ion exchange membranes under an electric field, without membrane pressure differential.
- Remove specific ions using state-of-the-art ion exchange membranes with 98% monovalent ionic selectivity (i.e. remove lithium, separate chlorides from sulfates).
- Change brine chemistry without softening to reach the full potential of your RO system and wring out even more freshwater for beneficial reuse.
- Concentrate brine to low volumes with membranes – territory previously reserved for evaporative systems.